Medical connectors
Fully customizable or off-the-shelf, our high-performance connectors are designed for demanding medical environments, from diagnostic and imaging to surgical, therapeutic and dental healthcare.



Our wide range of circular connectors – including miniature, sterilizable, reusable, hybrid, disposable and silicone-coated options – meet the strictest quality and reliability requirements in medical device manufacturing.
Fischer Connectors has a proven reputation for designing safe, sterilizable and high-performance solutions which help optimize the functionality of life-saving devices and ensure their electrical safety and protection.
Our wide range of circular connectors – including miniature, sterilizable, reusable, hybrid, disposable and silicone-coated options – meet the strictest quality and reliability requirements in medical device manufacturing.
Fischer Connectors has a proven reputation for designing safe, sterilizable and high-performance solutions which help optimize the functionality of life-saving devices and ensure their electrical safety and protection.
Our wide range of circular connectors – including miniature, sterilizable, reusable, hybrid, disposable and silicone-coated options – meet the strictest quality and reliability requirements in medical device manufacturing.
Fischer Connectors has a proven reputation for designing safe, sterilizable and high-performance solutions which help optimize the functionality of life-saving devices and ensure their electrical safety and protection.



Our wide range of circular connectors – including miniature, sterilizable, reusable, hybrid, disposable and silicone-coated options – meet the strictest quality and reliability requirements in medical device manufacturing.
Fischer Connectors has a proven reputation for designing safe, sterilizable and high-performance solutions which help optimize the functionality of life-saving devices and ensure their electrical safety and protection.
Our wide range of circular connectors – including miniature, sterilizable, reusable, hybrid, disposable and silicone-coated options – meet the strictest quality and reliability requirements in medical device manufacturing.
Fischer Connectors has a proven reputation for designing safe, sterilizable and high-performance solutions which help optimize the functionality of life-saving devices and ensure their electrical safety and protection.
Our wide range of circular connectors – including miniature, sterilizable, reusable, hybrid, disposable and silicone-coated options – meet the strictest quality and reliability requirements in medical device manufacturing.
Fischer Connectors has a proven reputation for designing safe, sterilizable and high-performance solutions which help optimize the functionality of life-saving devices and ensure their electrical safety and protection.



Our wide range of circular connectors – including miniature, sterilizable, reusable, hybrid, disposable and silicone-coated options – meet the strictest quality and reliability requirements in medical device manufacturing.
Fischer Connectors has a proven reputation for designing safe, sterilizable and high-performance solutions which help optimize the functionality of life-saving devices and ensure their electrical safety and protection.
Our wide range of circular connectors – including miniature, sterilizable, reusable, hybrid, disposable and silicone-coated options – meet the strictest quality and reliability requirements in medical device manufacturing.
Fischer Connectors has a proven reputation for designing safe, sterilizable and high-performance solutions which help optimize the functionality of life-saving devices and ensure their electrical safety and protection.
Our wide range of circular connectors – including miniature, sterilizable, reusable, hybrid, disposable and silicone-coated options – meet the strictest quality and reliability requirements in medical device manufacturing.
Fischer Connectors has a proven reputation for designing safe, sterilizable and high-performance solutions which help optimize the functionality of life-saving devices and ensure their electrical safety and protection.
Sales request
Our medical connector solutions
Fischer Connectors’ solutions for medical devices are well-known for their reliability, long lifespan and durability. Our medical connectors help customers overcome the challenge of developing ever smaller, lighter devices to create mobile and less invasive interactions with patients. Available solutions include various hybrid configurations that optimize size and weight by combining data, power and even gas or fluids in a single high-density, multi-tasking cable/connector.
The safety of medical patients is a primary concern for us, and we are committed to developing and providing products, services, and solutions that meet the highest industry standards. Our organization is certified to ISO 13485:2016 for the quality management of products that are safe and reliable for integration into medical devices, and we offer products and solutions that are compliant with IEC 60601-1 for patient and operator protection (MOPP/MOOP).
Fischer Connectors’ solutions for medical devices are well-known for their reliability, long lifespan and durability. Our medical connectors help customers overcome the challenge of developing ever smaller, lighter devices to create mobile and less invasive interactions with patients. Available solutions include various hybrid configurations that optimize size and weight by combining data, power and even gas or fluids in a single high-density, multi-tasking cable/connector.
The safety of medical patients is a primary concern for us, and we are committed to developing and providing products, services, and solutions that meet the highest industry standards. Our organization is certified to ISO 13485:2016 for the quality management of products that are safe and reliable for integration into medical devices, and we offer products and solutions that are compliant with IEC 60601-1 for patient and operator protection (MOPP/MOOP).
Fischer Connectors’ solutions for medical devices are well-known for their reliability, long lifespan and durability. Our medical connectors help customers overcome the challenge of developing ever smaller, lighter devices to create mobile and less invasive interactions with patients. Available solutions include various hybrid configurations that optimize size and weight by combining data, power and even gas or fluids in a single high-density, multi-tasking cable/connector.
The safety of medical patients is a primary concern for us, and we are committed to developing and providing products, services, and solutions that meet the highest industry standards. Our organization is certified to ISO 13485:2016 for the quality management of products that are safe and reliable for integration into medical devices, and we offer products and solutions that are compliant with IEC 60601-1 for patient and operator protection (MOPP/MOOP).
Key features
- Sterilization proof: Autocave (500 cycles minimum), Cidex, EtO, Gamma radiation, Steris® or Sterrad®
- IP68 sealing, resistance to heat and chemicals
- Robust design with mechanical endurance of 10’000 mating cycles minimum
- Highly configurable with a wide choice of high-performance materials (brass, stainless steel, plastic, PEEK) and customization capabilities
- Miniature and lightweight options
- Friction-free and flexible silicone overmolding
- Ergonomic and easy to handle and clean
- Combination of power and data, gas or fluids
- Optimized signal integrity
Key features
- Sterilization proof: Autocave (500 cycles minimum), Cidex, EtO, Gamma radiation, Steris® or Sterrad®
- IP68 sealing, resistance to heat and chemicals
- Robust design with mechanical endurance of 10’000 mating cycles minimum
- Highly configurable with a wide choice of high-performance materials (brass, stainless steel, plastic, PEEK) and customization capabilities
- Miniature and lightweight options
- Friction-free and flexible silicone overmolding
- Ergonomic and easy to handle and clean
- Combination of power and data, gas or fluids
- Optimized signal integrity
Key features
- Sterilization proof: Autocave (500 cycles minimum), Cidex, EtO, Gamma radiation, Steris® or Sterrad®
- IP68 sealing, resistance to heat and chemicals
- Robust design with mechanical endurance of 10’000 mating cycles minimum
- Highly configurable with a wide choice of high-performance materials (brass, stainless steel, plastic, PEEK) and customization capabilities
- Miniature and lightweight options
- Friction-free and flexible silicone overmolding
- Ergonomic and easy to handle and clean
- Combination of power and data, gas or fluids
- Optimized signal integrity



Our product range
Our product range
Our product range

Our custom connector capabilities will help you meet your needs.

Our custom connector capabilities will help you meet your needs.

Our custom connector capabilities will help you meet your needs.
Learn more about medical connectors
Medtech designers face some of the most stringent requirements in the world. Everything must be safe for the patient and safe for the operator. In addition, devices can be put through years of U.S. Food & Drug Administration (FDA) trials and trials in Europe, so reducing the risks associated with any device is paramount in the designer’s approach.
Along with ensuring a device is low risk, reliable, and functions to the highest expectations of a diverse base of medical professionals and their patients, manufacturers must meet another level of design challenges. Devices must look modern, provide a positive patient experience, include more technology and functionality in a smaller space.
Medical connectors and cable assemblies play an important role in all these trends, as they deliver power to hand tools and handheld diagnostic equipment and return signals to mobile consoles and surgical systems.
Selecting the right medical connector and cable assembly solution can help build small, safe, stylish, and functional devices that hit the right price point to keep manufacturers competitive while keeping risk low for both the patient and the operator.
Medtech designers face some of the most stringent requirements in the world. Everything must be safe for the patient and safe for the operator. In addition, devices can be put through years of U.S. Food & Drug Administration (FDA) trials and trials in Europe, so reducing the risks associated with any device is paramount in the designer’s approach.
Along with ensuring a device is low risk, reliable, and functions to the highest expectations of a diverse base of medical professionals and their patients, manufacturers must meet another level of design challenges. Devices must look modern, provide a positive patient experience, include more technology and functionality in a smaller space.
Medical connectors and cable assemblies play an important role in all these trends, as they deliver power to hand tools and handheld diagnostic equipment and return signals to mobile consoles and surgical systems.
Selecting the right medical connector and cable assembly solution can help build small, safe, stylish, and functional devices that hit the right price point to keep manufacturers competitive while keeping risk low for both the patient and the operator.
Medtech designers face some of the most stringent requirements in the world. Everything must be safe for the patient and safe for the operator. In addition, devices can be put through years of U.S. Food & Drug Administration (FDA) trials and trials in Europe, so reducing the risks associated with any device is paramount in the designer’s approach.
Along with ensuring a device is low risk, reliable, and functions to the highest expectations of a diverse base of medical professionals and their patients, manufacturers must meet another level of design challenges. Devices must look modern, provide a positive patient experience, include more technology and functionality in a smaller space.
Medical connectors and cable assemblies play an important role in all these trends, as they deliver power to hand tools and handheld diagnostic equipment and return signals to mobile consoles and surgical systems.
Selecting the right medical connector and cable assembly solution can help build small, safe, stylish, and functional devices that hit the right price point to keep manufacturers competitive while keeping risk low for both the patient and the operator.
Critical considerations for material choice, required sealing level and mating mechanism:
- How many mating cycles is the medical connector expected to last ?
- Who will operate, clean and sterilize the device: untrained patient or a trained medical professional ?
- What kind of conditions the device will need to endure ?
- Which sterilization method will be applied will be ?
The need to shield medical connectors from environmental hazards also includes shielding them from electromagnetic interference (EMI/RFI), which can introduce anomalies into data, cause a device to move when it shouldn’t, or prevent a device from operating. So many ordinary electronics emit EM energy that, depending on the medical device’s end use, it’s reasonable to plan on including EMI/RFI shielding to ensure reliable data transmission.
Critical considerations for material choice, required sealing level and mating mechanism:
- How many mating cycles is the medical connector expected to last ?
- Who will operate, clean and sterilize the device: untrained patient or a trained medical professional ?
- What kind of conditions the device will need to endure ?
- Which sterilization method will be applied will be ?
The need to shield medical connectors from environmental hazards also includes shielding them from electromagnetic interference (EMI/RFI), which can introduce anomalies into data, cause a device to move when it shouldn’t, or prevent a device from operating. So many ordinary electronics emit EM energy that, depending on the medical device’s end use, it’s reasonable to plan on including EMI/RFI shielding to ensure reliable data transmission.
Critical considerations for material choice, required sealing level and mating mechanism:
- How many mating cycles is the medical connector expected to last ?
- Who will operate, clean and sterilize the device: untrained patient or a trained medical professional ?
- What kind of conditions the device will need to endure ?
- Which sterilization method will be applied will be ?
The need to shield medical connectors from environmental hazards also includes shielding them from electromagnetic interference (EMI/RFI), which can introduce anomalies into data, cause a device to move when it shouldn’t, or prevent a device from operating. So many ordinary electronics emit EM energy that, depending on the medical device’s end use, it’s reasonable to plan on including EMI/RFI shielding to ensure reliable data transmission.
| Connector Size | Take a close look at who will be managing the connection. A patient who is sick and self-managing will have different needs than a caregiver or medical professional. Make sure your connector is small enough to help keep design in line, but large enough for anyone to get a good grip on it. In addition, keep an eye on the profile of the connector. The lower, the better when it comes to wearables. |
| Connections for Patient Mobility | If you are looking at mobility, look at how and how much the patient will be moving. Lots of mobility will mean a different set of design considerations than if a person is bedridden. New options for the most mobile patients include connectors with concentric rings that allow movement of the cable, making it less likely to be damaged over time. |
| Connector Torque | Many connectors are purposely designed so that it is hard to connect or disconnect. Make sure that the connector is easy enough to connect and disconnect, but difficult to accidently disconnect. |
| Shape & Locking Types | We admit to bias here, but we think circular push-pull connectors are perfect for wearables, with no little tabs to break off, rectangles to challenge your spatial awareness, or USB-type hassles when you can’t find the right way to place the connector into the receptacle no matter how many times you turn it over. Circular connectors also have quick-release/no lock solutions or if you need it to stay in place in high vibrations, look for a screw lock. |
| Cable Management | Wearables and devices that move around a lot require pre-planning for cable length and how the cables need to move with the person or the device. Think about sewing cables into clothing to minimize a patent’s ability to damage or tangle them. Make sure there is enough (but not too much) cable. You may want to consider offering different lengths of cable. Robotic devices and exoskeletons with embedded sensors also need protected cable systems. |
| EMI Shielding | The more technology in the area, the more you need EMI shielding to protect the system from the noise. But you can’t skip EMI protections just because a product will have in-home use. Medical devices are critical, and you can’t predict electrical interference on that alone. |
| Color | The more connectors, the more color-coding you want to consider. Minimize the number of colors needed with multi-use connectors for signal and power or air and signal. Look at materials that can be ordered in different colors, or rings that match connector or cable colors. |
| Connector Size | Take a close look at who will be managing the connection. A patient who is sick and self-managing will have different needs than a caregiver or medical professional. Make sure your connector is small enough to help keep design in line, but large enough for anyone to get a good grip on it. In addition, keep an eye on the profile of the connector. The lower, the better when it comes to wearables. |
| Connections for Patient Mobility | If you are looking at mobility, look at how and how much the patient will be moving. Lots of mobility will mean a different set of design considerations than if a person is bedridden. New options for the most mobile patients include connectors with concentric rings that allow movement of the cable, making it less likely to be damaged over time. |
| Connector Torque | Many connectors are purposely designed so that it is hard to connect or disconnect. Make sure that the connector is easy enough to connect and disconnect, but difficult to accidently disconnect. |
| Shape & Locking Types | We admit to bias here, but we think circular push-pull connectors are perfect for wearables, with no little tabs to break off, rectangles to challenge your spatial awareness, or USB-type hassles when you can’t find the right way to place the connector into the receptacle no matter how many times you turn it over. Circular connectors also have quick-release/no lock solutions or if you need it to stay in place in high vibrations, look for a screw lock. |
| Cable Management | Wearables and devices that move around a lot require pre-planning for cable length and how the cables need to move with the person or the device. Think about sewing cables into clothing to minimize a patent’s ability to damage or tangle them. Make sure there is enough (but not too much) cable. You may want to consider offering different lengths of cable. Robotic devices and exoskeletons with embedded sensors also need protected cable systems. |
| EMI Shielding | The more technology in the area, the more you need EMI shielding to protect the system from the noise. But you can’t skip EMI protections just because a product will have in-home use. Medical devices are critical, and you can’t predict electrical interference on that alone. |
| Color | The more connectors, the more color-coding you want to consider. Minimize the number of colors needed with multi-use connectors for signal and power or air and signal. Look at materials that can be ordered in different colors, or rings that match connector or cable colors. |
| Connector Size | Take a close look at who will be managing the connection. A patient who is sick and self-managing will have different needs than a caregiver or medical professional. Make sure your connector is small enough to help keep design in line, but large enough for anyone to get a good grip on it. In addition, keep an eye on the profile of the connector. The lower, the better when it comes to wearables. |
| Connections for Patient Mobility | If you are looking at mobility, look at how and how much the patient will be moving. Lots of mobility will mean a different set of design considerations than if a person is bedridden. New options for the most mobile patients include connectors with concentric rings that allow movement of the cable, making it less likely to be damaged over time. |
| Connector Torque | Many connectors are purposely designed so that it is hard to connect or disconnect. Make sure that the connector is easy enough to connect and disconnect, but difficult to accidently disconnect. |
| Shape & Locking Types | We admit to bias here, but we think circular push-pull connectors are perfect for wearables, with no little tabs to break off, rectangles to challenge your spatial awareness, or USB-type hassles when you can’t find the right way to place the connector into the receptacle no matter how many times you turn it over. Circular connectors also have quick-release/no lock solutions or if you need it to stay in place in high vibrations, look for a screw lock. |
| Cable Management | Wearables and devices that move around a lot require pre-planning for cable length and how the cables need to move with the person or the device. Think about sewing cables into clothing to minimize a patent’s ability to damage or tangle them. Make sure there is enough (but not too much) cable. You may want to consider offering different lengths of cable. Robotic devices and exoskeletons with embedded sensors also need protected cable systems. |
| EMI Shielding | The more technology in the area, the more you need EMI shielding to protect the system from the noise. But you can’t skip EMI protections just because a product will have in-home use. Medical devices are critical, and you can’t predict electrical interference on that alone. |
| Color | The more connectors, the more color-coding you want to consider. Minimize the number of colors needed with multi-use connectors for signal and power or air and signal. Look at materials that can be ordered in different colors, or rings that match connector or cable colors. |
Safety
- ESD (electrostatic discharge) protection
- MOPP (Means of Patient Protection), MOOP (Means of Operator Protection) and compliance with test finger standard
- Air clearance and creepage distances
- Insulation barriers and insulated contacts
Safety
- ESD (electrostatic discharge) protection
- MOPP (Means of Patient Protection), MOOP (Means of Operator Protection) and compliance with test finger standard
- Air clearance and creepage distances
- Insulation barriers and insulated contacts
Safety
- ESD (electrostatic discharge) protection
- MOPP (Means of Patient Protection), MOOP (Means of Operator Protection) and compliance with test finger standard
- Air clearance and creepage distances
- Insulation barriers and insulated contacts
Signal integrity optimization
- Highs-speed multiprotocol data transfer
- Minimized noise and signal distortion
- Controlled cable assembly process for impedance matching
- Minimized near-end / far-end crosstalk (NEXT / FEXT)
Signal integrity optimization
- Highs-speed multiprotocol data transfer
- Minimized noise and signal distortion
- Controlled cable assembly process for impedance matching
- Minimized near-end / far-end crosstalk (NEXT / FEXT)
Signal integrity optimization
- Highs-speed multiprotocol data transfer
- Minimized noise and signal distortion
- Controlled cable assembly process for impedance matching
- Minimized near-end / far-end crosstalk (NEXT / FEXT)
Durability
- 500 sterilization cycles proven
- Resistant to sterilization and chemicals (autoclave, Cidex, EtO, Gamma radiation, Steris® or Sterrad®)
- Resistant to high-pressure steam (hydrogen peroxide vapor, Sterrad®)
- Thermal insulation
- Available with silicone overmolding
Durability
- 500 sterilization cycles proven
- Resistant to sterilization and chemicals (autoclave, Cidex, EtO, Gamma radiation, Steris® or Sterrad®)
- Resistant to high-pressure steam (hydrogen peroxide vapor, Sterrad®)
- Thermal insulation
- Available with silicone overmolding
Durability
- 500 sterilization cycles proven
- Resistant to sterilization and chemicals (autoclave, Cidex, EtO, Gamma radiation, Steris® or Sterrad®)
- Resistant to high-pressure steam (hydrogen peroxide vapor, Sterrad®)
- Thermal insulation
- Available with silicone overmolding
Custom designs
- Brass, stainless steel, plastic, PEEK
- Custom color coding and locking mechanisms
- Miniature and lightweight
- Ergonomic and easy to handle
- Hybrid connectivity – fluidic / optical / electrical
- Robust design with double shielding
Custom designs
- Brass, stainless steel, plastic, PEEK
- Custom color coding and locking mechanisms
- Miniature and lightweight
- Ergonomic and easy to handle
- Hybrid connectivity – fluidic / optical / electrical
- Robust design with double shielding
Custom designs
- Brass, stainless steel, plastic, PEEK
- Custom color coding and locking mechanisms
- Miniature and lightweight
- Ergonomic and easy to handle
- Hybrid connectivity – fluidic / optical / electrical
- Robust design with double shielding
Our spectrum of medical connector applications
Fischer Connectors is proud to be recognized as an essential partner for medical device manufacturing. With our high-reliability interconnect solutions, we serve the world’s leading medical organizations and suppliers of mission-critical devices, helping protect and save lives across a wide variety of medical domains and applications.
Fischer Connectors is proud to be recognized as an essential partner for medical device manufacturing. With our high-reliability interconnect solutions, we serve the world’s leading medical organizations and suppliers of mission-critical devices, helping protect and save lives across a wide variety of medical domains and applications.
Fischer Connectors is proud to be recognized as an essential partner for medical device manufacturing. With our high-reliability interconnect solutions, we serve the world’s leading medical organizations and suppliers of mission-critical devices, helping protect and save lives across a wide variety of medical domains and applications.
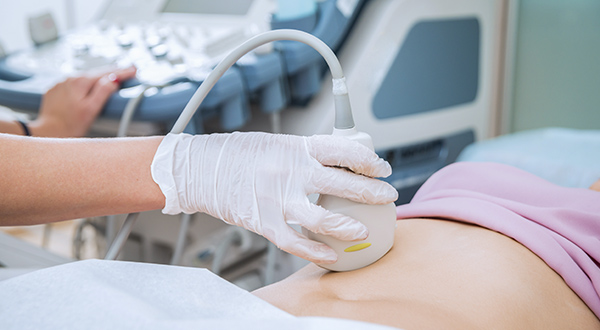


Diagnostic & Imaging
Fischer Connectors provides dependable, high-speed connectivity solutions for accurate imaging and diagnostics, e.g., endoscopy, laparoscopy, electroencephalography (EEG), MRI and CT scanners or medical ultrasound.
To perform safe diagnostic procedures, for example with reusable endoscopes, the connectivity solution must be hermetically sealed to protect electronics from cleaning, disinfection and reprocessing procedures, which expose these devices and components to harsh chemicals and challenging environments such as autoclave (steam sterilization) or Steris and Sterrad (low-temperature sterilization) systems. The use of durable medical connectors with metal shells and cables with silicone overmolding is recommended to enable the device to endure between 250 and 1,000 cycles according to reprocessing guidelines.
Fischer Connectors offers a wide range of sizes, configurations and coding options for medical connectors which withstand extreme sterilization processes and feature more than 5,000 mating cycles.
Diagnostic & Imaging
Fischer Connectors provides dependable, high-speed connectivity solutions for accurate imaging and diagnostics, e.g., endoscopy, laparoscopy, electroencephalography (EEG), MRI and CT scanners or medical ultrasound.
To perform safe diagnostic procedures, for example with reusable endoscopes, the connectivity solution must be hermetically sealed to protect electronics from cleaning, disinfection and reprocessing procedures, which expose these devices and components to harsh chemicals and challenging environments such as autoclave (steam sterilization) or Steris and Sterrad (low-temperature sterilization) systems. The use of durable medical connectors with metal shells and cables with silicone overmolding is recommended to enable the device to endure between 250 and 1,000 cycles according to reprocessing guidelines.
Fischer Connectors offers a wide range of sizes, configurations and coding options for medical connectors which withstand extreme sterilization processes and feature more than 5,000 mating cycles.
Diagnostic & Imaging
Fischer Connectors provides dependable, high-speed connectivity solutions for accurate imaging and diagnostics, e.g., endoscopy, laparoscopy, electroencephalography (EEG), MRI and CT scanners or medical ultrasound.
To perform safe diagnostic procedures, for example with reusable endoscopes, the connectivity solution must be hermetically sealed to protect electronics from cleaning, disinfection and reprocessing procedures, which expose these devices and components to harsh chemicals and challenging environments such as autoclave (steam sterilization) or Steris and Sterrad (low-temperature sterilization) systems. The use of durable medical connectors with metal shells and cables with silicone overmolding is recommended to enable the device to endure between 250 and 1,000 cycles according to reprocessing guidelines.
Fischer Connectors offers a wide range of sizes, configurations and coding options for medical connectors which withstand extreme sterilization processes and feature more than 5,000 mating cycles.
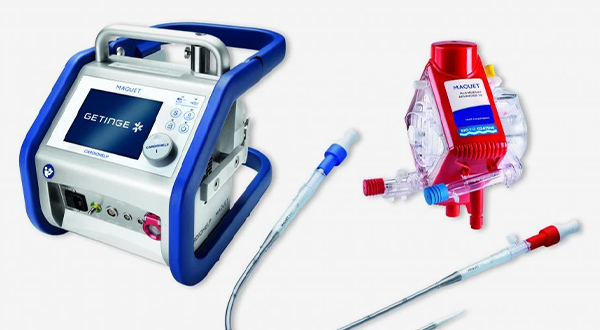


Monitoring
Our medical connectors feature reliable and straightforward connectivity technology for vital patient information, critical care and rescue operations, e.g., Getinge’s Cardiohelp. This mobile heart-lung support system is easy to use and quick to set up in a wide range of indications in cardiac surgery, cardiology, intensive care and emergency medicine.
The manufacturer’s primary considerations for selecting the space-saving Fischer Core connectors used in this compact system were their exceptional durability, high-quality mating capabilities, reliable signal transmission, and user-friendliness. Additionally, the medical connectors needed to be both easy to clean and robust enough to withstand harsh medical cleaning agents without sustaining any damage.
Monitoring
Our medical connectors feature reliable and straightforward connectivity technology for vital patient information, critical care and rescue operations, e.g., Getinge’s Cardiohelp. This mobile heart-lung support system is easy to use and quick to set up in a wide range of indications in cardiac surgery, cardiology, intensive care and emergency medicine.
The manufacturer’s primary considerations for selecting the space-saving Fischer Core connectors used in this compact system were their exceptional durability, high-quality mating capabilities, reliable signal transmission, and user-friendliness. Additionally, the medical connectors needed to be both easy to clean and robust enough to withstand harsh medical cleaning agents without sustaining any damage.
Monitoring
Our medical connectors feature reliable and straightforward connectivity technology for vital patient information, critical care and rescue operations, e.g., Getinge’s Cardiohelp. This mobile heart-lung support system is easy to use and quick to set up in a wide range of indications in cardiac surgery, cardiology, intensive care and emergency medicine.
The manufacturer’s primary considerations for selecting the space-saving Fischer Core connectors used in this compact system were their exceptional durability, high-quality mating capabilities, reliable signal transmission, and user-friendliness. Additionally, the medical connectors needed to be both easy to clean and robust enough to withstand harsh medical cleaning agents without sustaining any damage.
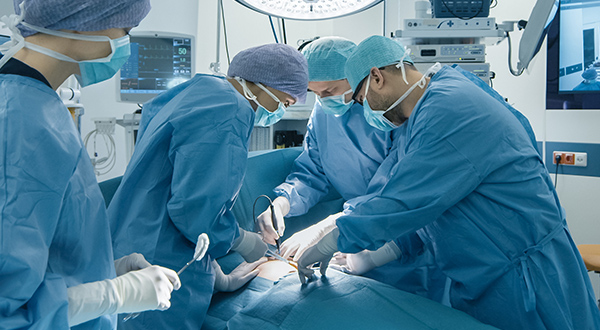


Interventional & Surgical
At Fischer Connectors, we understand the importance of secure and durable connectivity solutions adapted to high-precision devices and equipment in robot-assisted surgery, dental surgery, shaver systems, or power consoles and powered instrument drivers for interventional and surgical procedures.
Our medical connectors are designed to withstand the rigorous demands of surgical applications, with features such as hermetic sealing, high mating cycles, and resistance to harsh cleaning agents or sterilization procedures, while ensuring signal integrity for reliable data transmission. The flexibility in design and manufacturing ensures that our medical connectors can be seamlessly integrated into medical devices and equipment for improved performance and efficiency.
Interventional & Surgical
At Fischer Connectors, we understand the importance of secure and durable connectivity solutions adapted to high-precision devices and equipment in robot-assisted surgery, dental surgery, shaver systems, or power consoles and powered instrument drivers for interventional and surgical procedures.
Our medical connectors are designed to withstand the rigorous demands of surgical applications, with features such as hermetic sealing, high mating cycles, and resistance to harsh cleaning agents or sterilization procedures, while ensuring signal integrity for reliable data transmission. The flexibility in design and manufacturing ensures that our medical connectors can be seamlessly integrated into medical devices and equipment for improved performance and efficiency.
Interventional & Surgical
At Fischer Connectors, we understand the importance of secure and durable connectivity solutions adapted to high-precision devices and equipment in robot-assisted surgery, dental surgery, shaver systems, or power consoles and powered instrument drivers for interventional and surgical procedures.
Our medical connectors are designed to withstand the rigorous demands of surgical applications, with features such as hermetic sealing, high mating cycles, and resistance to harsh cleaning agents or sterilization procedures, while ensuring signal integrity for reliable data transmission. The flexibility in design and manufacturing ensures that our medical connectors can be seamlessly integrated into medical devices and equipment for improved performance and efficiency.
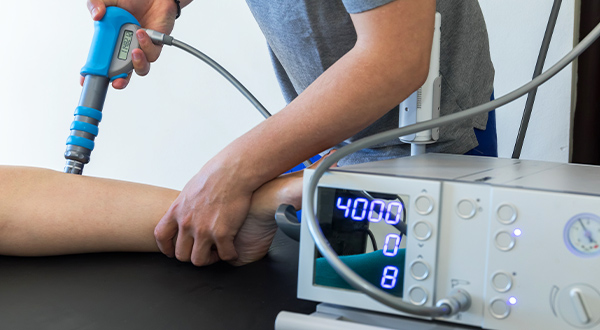


Therapeutic
Our connectivity solutions are widely used in therapeutic devices. Examples can be found in shock-wave medical applications which require robust, durable and reliable connectors, easy to clean and sterilizable. Our medical connectors are designed to withstand the demanding conditions of such medical equipment, including exposure to shock and vibration.
They provide reliable signal transmission and ensure secure mating, ensuring that the shock wave machine functions optimally without any disruptions or malfunctions.
Therapeutic
Our connectivity solutions are widely used in therapeutic devices. Examples can be found in shock-wave medical applications which require robust, durable and reliable connectors, easy to clean and sterilizable. Our medical connectors are designed to withstand the demanding conditions of such medical equipment, including exposure to shock and vibration.
They provide reliable signal transmission and ensure secure mating, ensuring that the shock wave machine functions optimally without any disruptions or malfunctions.
Therapeutic
Our connectivity solutions are widely used in therapeutic devices. Examples can be found in shock-wave medical applications which require robust, durable and reliable connectors, easy to clean and sterilizable. Our medical connectors are designed to withstand the demanding conditions of such medical equipment, including exposure to shock and vibration.
They provide reliable signal transmission and ensure secure mating, ensuring that the shock wave machine functions optimally without any disruptions or malfunctions.
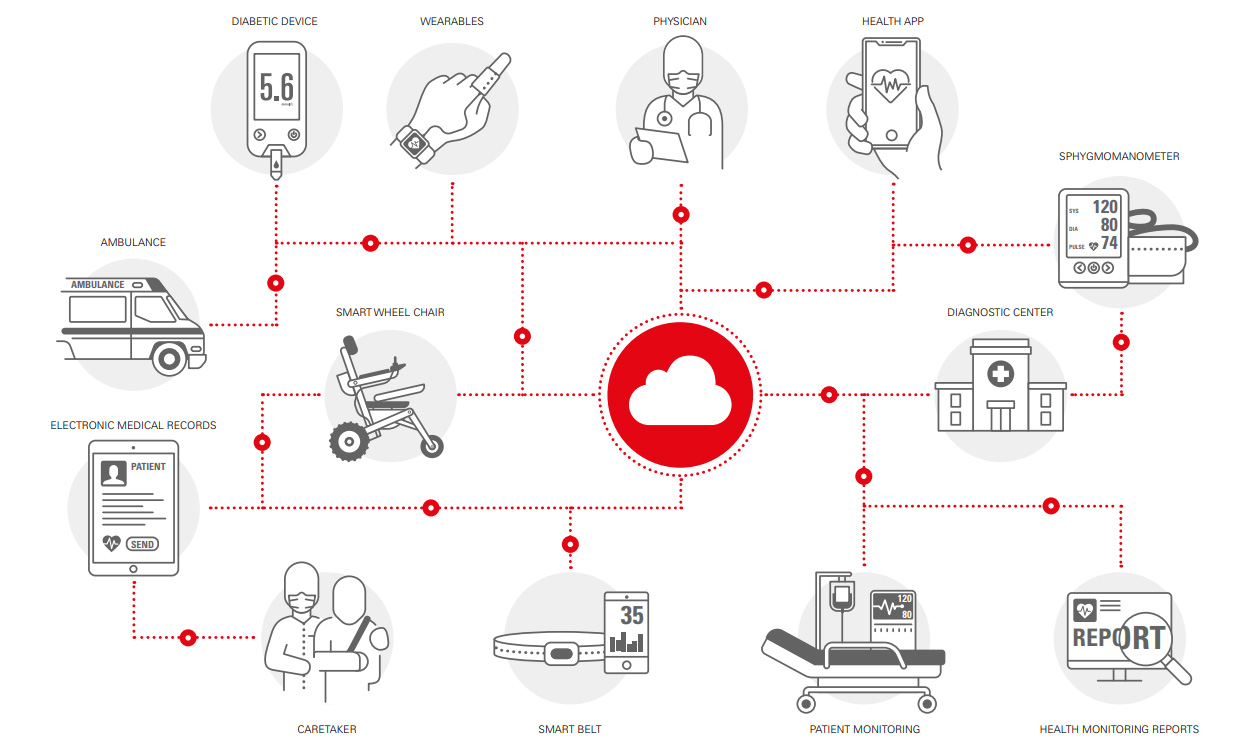
At Fischer Connectors, we offer fully integrated connectivity solutions to facilitate the implementation of the Internet of Medical Things (IoMT).
At Fischer Connectors, we offer fully integrated connectivity solutions to facilitate the implementation of the Internet of Medical Things (IoMT).
At Fischer Connectors, we offer fully integrated connectivity solutions to facilitate the implementation of the Internet of Medical Things (IoMT).
Medical device connectors – Miniaturization
Where miniaturization is succeeding is in medtech. The newest innovations that are revolutionizing healthcare and how it is delivered have successfully implemented designs that take advantage of the new rugged medical connectors that carry both signal and power. This movement continues to expand into some of the newest mobile devices, patient-worn devices that the patient monitors and manages, and new devices incorporating technology such as sensors that report back — sometimes wirelessly — to a station.
Designing tiny connectors comes with its own challenges. The choice of material, the number of pins, the size of each pin, and the amount of electricity each pin can handle will influence the creepage and air clearance needs, which affect how many pins can fit in a smaller space. A higher pin density allows a single small medical connector to do what would otherwise require multiple larger connectors, transmitting data and power together without interference.
Miniaturization of a connector solution depends not just on the connector, but also on the cable. It is imperative to test connector/cable pairs and match the connector with a cable that is the correct size, which may involve a custom cable to ensure it fits the small medical connector while remaining free of interference.
Where miniaturization is succeeding is in medtech. The newest innovations that are revolutionizing healthcare and how it is delivered have successfully implemented designs that take advantage of the new rugged medical connectors that carry both signal and power. This movement continues to expand into some of the newest mobile devices, patient-worn devices that the patient monitors and manages, and new devices incorporating technology such as sensors that report back — sometimes wirelessly — to a station.
Designing tiny connectors comes with its own challenges. The choice of material, the number of pins, the size of each pin, and the amount of electricity each pin can handle will influence the creepage and air clearance needs, which affect how many pins can fit in a smaller space. A higher pin density allows a single small medical connector to do what would otherwise require multiple larger connectors, transmitting data and power together without interference.
Miniaturization of a connector solution depends not just on the connector, but also on the cable. It is imperative to test connector/cable pairs and match the connector with a cable that is the correct size, which may involve a custom cable to ensure it fits the small medical connector while remaining free of interference.
Where miniaturization is succeeding is in medtech. The newest innovations that are revolutionizing healthcare and how it is delivered have successfully implemented designs that take advantage of the new rugged medical connectors that carry both signal and power. This movement continues to expand into some of the newest mobile devices, patient-worn devices that the patient monitors and manages, and new devices incorporating technology such as sensors that report back — sometimes wirelessly — to a station.
Designing tiny connectors comes with its own challenges. The choice of material, the number of pins, the size of each pin, and the amount of electricity each pin can handle will influence the creepage and air clearance needs, which affect how many pins can fit in a smaller space. A higher pin density allows a single small medical connector to do what would otherwise require multiple larger connectors, transmitting data and power together without interference.
Miniaturization of a connector solution depends not just on the connector, but also on the cable. It is imperative to test connector/cable pairs and match the connector with a cable that is the correct size, which may involve a custom cable to ensure it fits the small medical connector while remaining free of interference.
| Device type | Connector Consideration | Cable Consideration |
| Surgical hand tools |
|
Must be sterilizable. Consider high-heat silicone with a low friction coating. |
| Dental hand tools |
|
Lightweight, but small and strong enough to handle the power required |
| Handheld diagnostic devices |
|
May be sterilizable. Color-code overmolding for ease of use Lightweight cable with high EMI protection |
| Patient Care and Monitoring (home or office care) |
|
Color-coded cable and overmolds when multiple connectors are in use |
| Surgical robotics |
|
High degree of flexibility |
| Portable medical consoles |
|
Effective high-speed communication via required protocol |
| Imaging devices |
|
High speed video data cables often required |
| Wearables |
|
Light, flexible cables. May consider a ribbon cable for a lower profile |
| Device type | Connector Consideration | Cable Consideration |
| Surgical hand tools |
|
Must be sterilizable. Consider high-heat silicone with a low friction coating. |
| Dental hand tools |
|
Lightweight, but small and strong enough to handle the power required |
| Handheld diagnostic devices |
|
May be sterilizable. Color-code overmolding for ease of use Lightweight cable with high EMI protection |
| Patient Care and Monitoring (home or office care) |
|
Color-coded cable and overmolds when multiple connectors are in use |
| Surgical robotics |
|
High degree of flexibility |
| Portable medical consoles |
|
Effective high-speed communication via required protocol |
| Imaging devices |
|
High speed video data cables often required |
| Wearables |
|
Light, flexible cables. May consider a ribbon cable for a lower profile |
| Device type | Connector Consideration | Cable Consideration |
| Surgical hand tools |
|
Must be sterilizable. Consider high-heat silicone with a low friction coating. |
| Dental hand tools |
|
Lightweight, but small and strong enough to handle the power required |
| Handheld diagnostic devices |
|
May be sterilizable. Color-code overmolding for ease of use Lightweight cable with high EMI protection |
| Patient Care and Monitoring (home or office care) |
|
Color-coded cable and overmolds when multiple connectors are in use |
| Surgical robotics |
|
High degree of flexibility |
| Portable medical consoles |
|
Effective high-speed communication via required protocol |
| Imaging devices |
|
High speed video data cables often required |
| Wearables |
|
Light, flexible cables. May consider a ribbon cable for a lower profile |